Hypertension Research Study
Researchers at Stanford Medicine are leading the SPYRAL AFFIRM Clinical Study to assess a potential new treatment for high blood pressure that isn’t controlled by medication.
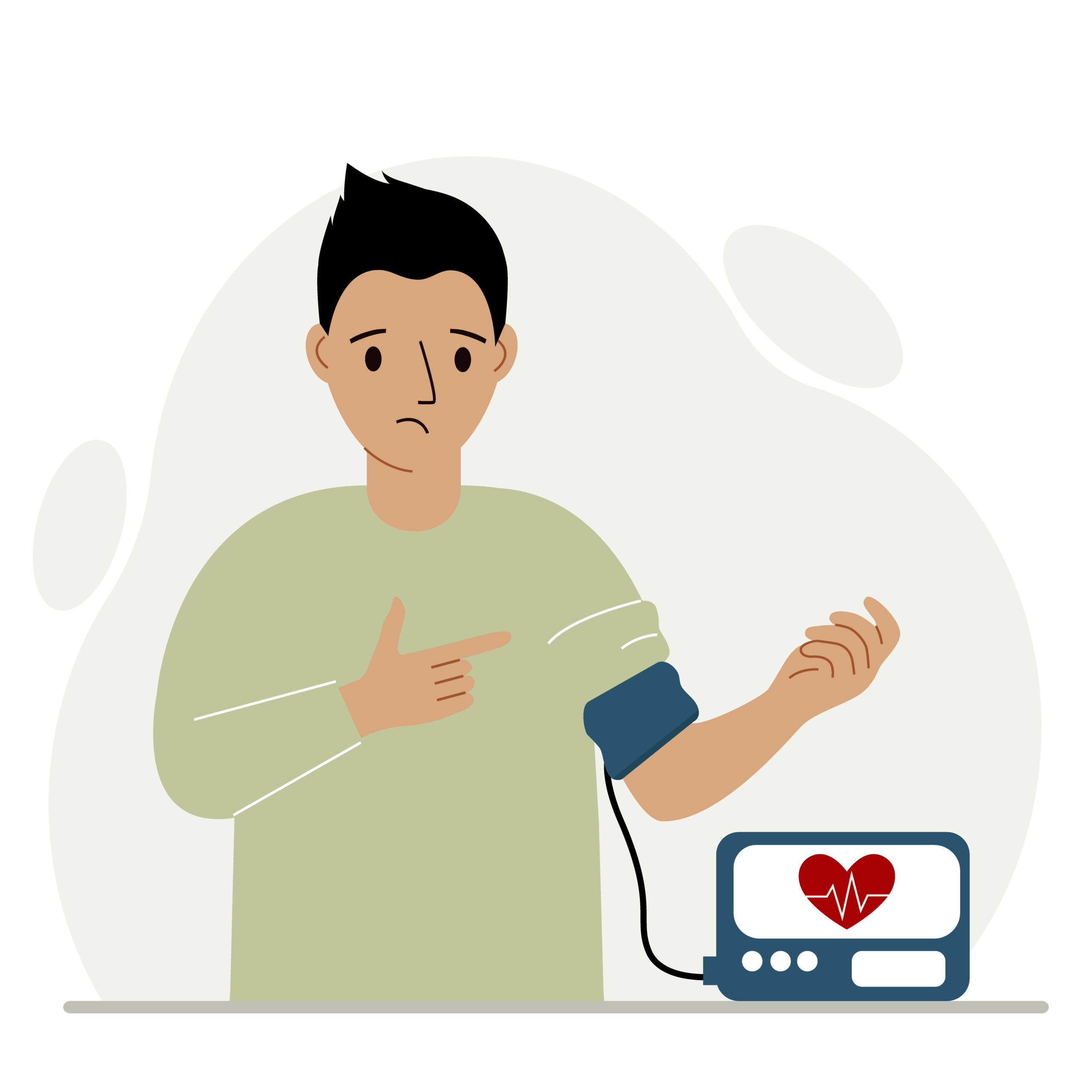
Fast Facts

Diagnosed with Hypertension (high blood pressure)

Non-White

Conducted in Palo Alto, CA
Study Background
Stanford Medicine is conducting the SPYRAL AFFIRM Clinical Study to evaluate a promising new treatment for high blood pressure that remains uncontrolled despite medication.
This study focuses on the safety, effectiveness, and long-term outcomes of a procedure called Renal Denervation (RDN) using the Symplicity Spyral™ System. RDN is a minimally invasive procedure that calms overactive nerves connected to the kidneys, which may be contributing to high blood pressure.
Participants will undergo the RDN procedure and attend follow-up visits to track blood pressure, medication usage, and overall health. Data collection will include blood pressure readings, safety assessments, and reporting of any side effects or health changes. This study also offers long-term follow-up for eligible participants from previous SPYRAL studies.
By joining the SPYRAL AFFIRM study, you can contribute to advancing research in hypertension treatment while potentially benefiting from an innovative approach to managing high blood pressure. If you have uncontrolled hypertension and are interested, contact Stanford Medicine for more information.

Study Background
Stanford Medicine is conducting the SPYRAL AFFIRM Clinical Study to evaluate a promising new treatment for high blood pressure that remains uncontrolled despite medication.

This study focuses on the safety, effectiveness, and long-term outcomes of a procedure called Renal Denervation (RDN) using the Symplicity Spyral™ System. RDN is a minimally invasive procedure that calms overactive nerves connected to the kidneys, which may be contributing to high blood pressure.
Participants will undergo the RDN procedure and attend follow-up visits to track blood pressure, medication usage, and overall health. Data collection will include blood pressure readings, safety assessments, and reporting of any side effects or health changes. This study also offers long-term follow-up for eligible participants from previous SPYRAL studies.
By joining the SPYRAL AFFIRM study, you can contribute to advancing research in hypertension treatment while potentially benefiting from an innovative approach to managing high blood pressure. If you have uncontrolled hypertension and are interested, contact Stanford Medicine for more information.

Additional Information
This study is being conducted to evaluate the safety, effectiveness, and long-term impact of the Symplicity Spyral™ Renal Denervation (RDN) System as a treatment for high blood pressure that remains uncontrolled despite medication. The procedure targets overactive nerves connected to the kidneys, which may contribute to hypertension. By studying this approach, researchers aim to determine if RDN can provide a durable and effective solution for managing high blood pressure when traditional treatments fall short.
You may qualify for a study if you meet the following criteria.
Inclusion Criteria:
-
Non-White
-
Diagnosed with hypertension (high blood pressure)
-
High blood pressure has not been adequately managed with lifestyle changes and medications
-
Have not undergone renal denervation procedure
-
Do not have conditions that make it hard. to measure blood pressure accurately
-
Do not use oxygen or a ventilator, except for sleep apnea at night
-
Kidney function (eGFR) is not below 45
-
Blood pressure does not drop when standing up (orthostatic hypotension)
-
Not diagnosed with primary pulmonary hypertension (a condition where blood pressure in the lungs’ arteries is abnormally high)
-
Not pregnant, breastfeeding, or planning to become pregnant in the near future
-
No diagnosis of type 1 diabetes or uncontrolled type 2 diabetes
If you participate in the study, you will undergo the Renal Denervation (RDN) procedure using the Symplicity Spyral™ System. This minimally invasive procedure targets overactive nerves connected to the kidneys that may contribute to your high blood pressure. After the procedure, you will attend scheduled follow-up visits where researchers will monitor your blood pressure, medication usage, and overall health. You’ll be asked to provide blood pressure readings, participate in safety assessments, and report any side effects or changes in your health. This helps researchers evaluate the effectiveness and safety of the treatment over time.
Compensation is not provided.
There is no cost for you to participate in our research study.
