Keloid Treatment Study
Researchers at BIDMC are partnering with individuals who have keloids (thick raised scars) to evaluate whether dupilumab can help. Join our compensated study today!

Fast Facts
18-65 years old
one keloid 2+ cm or two keloids 0.4+ cm
no history of HIV, hep b, hep c, or TB
Compensation Provided
Conducted in Boston, MA
Study Background
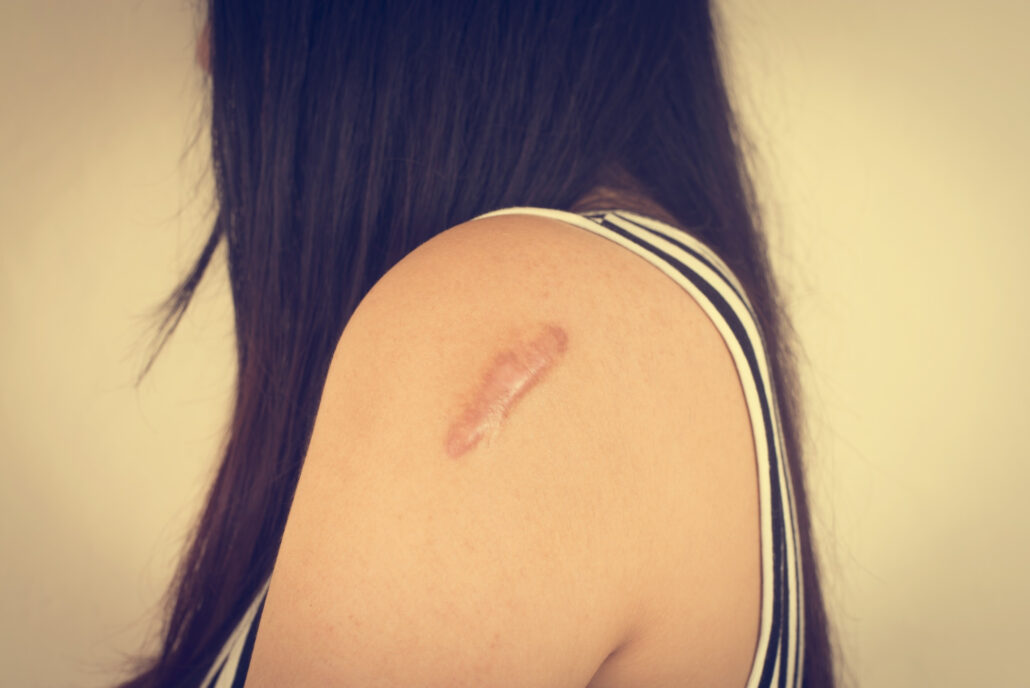
Keloids are thick, raised scars that form after a wound has healed and can be much larger than the original injury. The purpose of this study is to assess the effectiveness and safety of a medication called dupilumab (Dupixent) in the treatment of keloids.
Your participation in this study may help researchers improve treatment approaches for keloid scars. Further research today and join our compensated study!
Study Background
Keloids are thick, raised scars that form after a wound has healed and can be much larger than the original injury. The purpose of this study is to assess the effectiveness and safety of a medication called dupilumab (Dupixent) in the treatment of keloids.
Your participation in this study may help researchers improve treatment approaches for keloid scars. Further research today and join our compensated study!
Additional Information
To learn more about keloids, please reference this website: https://www.aad.org/public/diseases/a-z/264-symptoms
You may qualify for this study if you meet the following criteria.
Key Criteria:
- 18-65 years old
- One keloid at least 2 cm long, OR two or more keloids that are at least 0.4 wide x 0.4 cm long
- No history of HIV, hepatitis B, hepatitis C, or tuberculosis
- Not pregnant, breastfeeding, or planning to become pregnant during the study
- Not taking other immunosuppressants
- No acute asthma, acute bronchospasm, or status asthmaticus
- Have not participated in another study in the last month
- No treatment for your keloid(s) in the past month
- No history of cancer
- No history of organ transplant
The study medication, dupilumab, is taken as an injection under the skin. Study visits will occur about every 4 weeks over the course of 6 months. There may also be up to 2 biopsy visits.



